Nanoreactors mimicing plant cells
Living cells are a hive of activity, full of tiny structures making proteins, breaking down junk, and creating energy. All of this happens through a series of chemical reactions made possible largely because of the humble cell wall. “If you remove the cell wall, keeping exactly the same molecules that were inside the cell, then the same reactions will not occur or will occur very, very, slowly,“ says Cecile Malardier-Jugroot, Associate Professor in chemistry and chemical engineering at the Royal Military College of Canada.
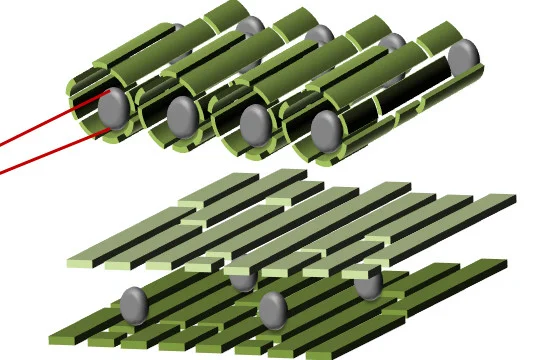
Inspired by the reactive power of cells, her team and collaborators are working to develop nanoreactors that mimic natural biosystems and their very high efficiency within a confined space. Their hope is to produce something more stable than biosystems offer, harnessing their advantages and using their structure and function as a model.
By introducing confinement to nanosystems, researchers mimic the effect of the cell wall on reactions. Suddenly, reactions that would not occur normally in bulk materials start to form. They’re fairly slow reactions, but they are special because no heat, pressure, nor catalysts need to be added to the mix to get the activity going.
Biosystems are inherently efficient in both thermodynamic and kinetic terms, but how best to maximize their nanoreactor cousins will take a great deal of study. The goal is to make more efficient fuel cells than anything currently on the market, working with carbon nanotubes and sheets, on the scale of individual atoms.
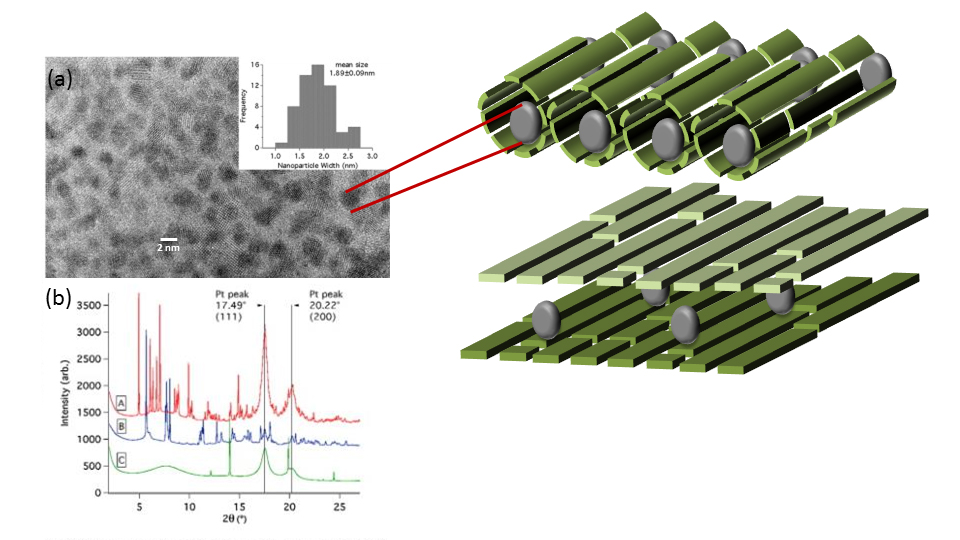
One of the first variables to test is the introduction of platinum crystals within their nanoreactor templates — a design also inspired by nature, since many cells use a metal centre as a reactive site. This first nanoreactor provided a 3 fold increase in the reaction rate within the template in the presence of the metal center compare to the empty nanoreactor. The researchers also plan to test gold and gold-platinum alloys to open up more potential applications.
The function of these metal-reactive site systems is still relatively new territory, and called for new research methods.
To achieve the fine detail needed to analyze these systems, the team used the Canadian Light Source synchrotron, transmission electron microscopy, and the Canadian Centre for Electron Microscopy.
“When we started this four years ago, we did the characterization using transmission electron microscopy, however with this technique, the samples have to be in the dry state and you get only the sense of one part of the sample, not the average of the sample which we needed to find out if we had the same number of clusters distributed everywhere in our sample.”
The Malardier-Jugroot team is most specialized in modelling, so they were grateful to work with the CLS crystallography scientists, who performed the X-Ray Diffraction analysis for the researchers.
“We’re trying to understand at a the atomic level what’s happening inside the nanoreactor templates, so having a link with experimentalists at that level, and being able to send them our models and trying to understand the interactions going on — that was very very helpful.”
There were surprises in store. For one thing, the templates, which were normally only stable at pH 7, remained stable from pH 3-13 with the addition of platinum. How and why that occurs is a major question.
In fact, the field remains full of mysteries: the nanoreactors can be light sensitive, so how can that be harnessed? What creates specific crystal structures? How do these structures affect function? Fuel cells are only one potential application for these mimic of biosystems — more wait to be uncovered.